MARKET TRENDS
Gene Editing’s Next Battle: Cracking the Delivery Code
Biotech firms like Intellia and Editas race to crack CRISPR delivery, a key to market dominance and future gene therapy breakthroughs.
7 Aug 2025
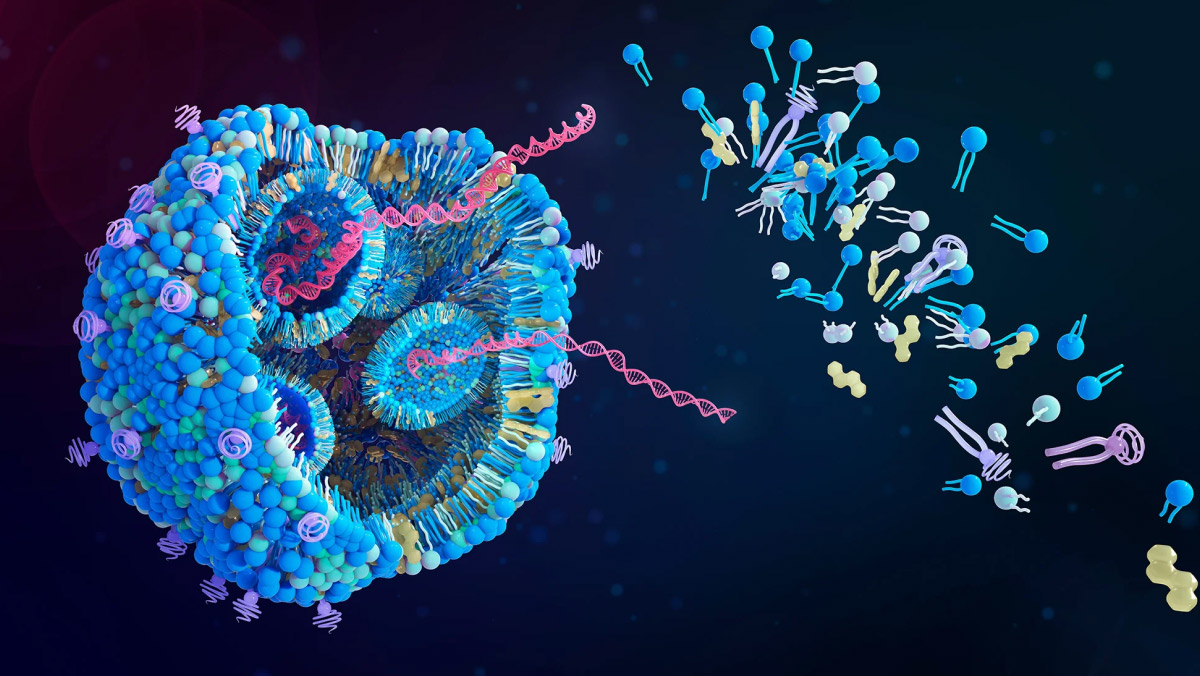
The gene editing sector is entering a new phase as companies move beyond improving CRISPR’s editing accuracy to focus on delivering therapies directly to target tissues. Delivery technology has become central to determining which firms will lead the next stage of genetic medicine.
Once a technical afterthought, delivery systems such as lipid nanoparticles and engineered viral vectors are now seen as critical assets. Their ability to carry gene-editing tools safely and efficiently into the body is shaping corporate strategy, investment flows and licensing deals across the industry.
Intellia Therapeutics is developing proprietary delivery methods that allow CRISPR therapies to be administered into the bloodstream and directed to the liver, enabling in vivo correction of genetic mutations. Early trial data have attracted interest from pharmaceutical partners, raising expectations for treatments that can be manufactured and deployed at scale.
Editas Medicine is pursuing a similar shift. The company is advancing in vivo delivery platforms and engaging with regulators on approaches that would allow platform-based approvals. Recent proof-of-concept results in its laboratories have given the company confidence to address safety and efficacy issues earlier in development.
Industry observers say these capabilities are becoming decisive. “There’s a growing realization that delivery isn’t just an engineering problem; it’s a commercial advantage,” said one analyst following the sector.
The shift comes amid heightened regulatory scrutiny over immune reactions and long-term safety risks. Reliable delivery platforms are seen as key to overcoming these concerns. At the same time, intellectual property disputes over delivery technologies are emerging as a source of competitive tension.
More than a dozen US biotech companies are now investing heavily in delivery research, according to industry estimates, reflecting a broader realignment of resources. For many executives and investors, success in the field will depend not only on editing genetic code, but on ensuring it reaches the right cells safely and consistently.
Latest News
15 Jan 2026
FDA opens smoother lanes for CRISPR firms13 Jan 2026
CRISPR tries to crack the rare-disease problem9 Jan 2026
Why Gene Editing Suddenly Feels Inevitable16 Dec 2025
A 2021 Gene Editing Deal Still Shapes the Future of Cancer Care
Related News

REGULATORY
15 Jan 2026
FDA opens smoother lanes for CRISPR firms
PARTNERSHIPS
13 Jan 2026
CRISPR tries to crack the rare-disease problem

INNOVATION
9 Jan 2026
Why Gene Editing Suddenly Feels Inevitable
SUBSCRIBE FOR UPDATES
By submitting, you agree to receive email communications from the event organizers, including upcoming promotions and discounted tickets, news, and access to related events.